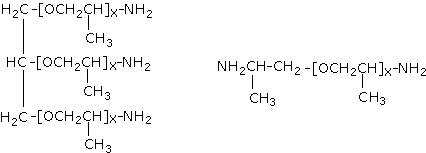
Traditionally, acrylic resins are used in paints and coatings. Acrylic polyols are employed in PU coatings (Chapter 7) for automotive finish with good chemical resistance and durability. In Latin America these polyols are consumed at an annual rate of 3,000 tons. They are obtained from the copolymerization of conventional acrylic monomers, such as ethyl acrylates (EA) or butyl acrylates (BA), acrylic acid (AA), methyl methacrylate (MMA), or styrene (ST) with hydroxylated acrylic monomers such as 2-hydroxyethyl acrylates (HEA) or 4-hydroxybutyl acrylates (HBA). The increase in styrene content makes the acrylic polyol more hydrophobic, while the use of HBA makes it more reactive due to lower steric hindrance in the hydroxyl group. Usually, acrylic polyols used in solvent-based 2K-PUR systems are acrylic resins with OH content (based on solid resin) of 0.5 to 3.5%, equivalent weights from 3,400 to 500, non-volatile content from 40 to 100%, in solvents as xylene (X), naphtha (N), or butyl acetate (BA), or 1-methoxypropyl acetate (MPA), and viscosities between 1,800 and 9,000 mPa.s. Aqueous 2K-PUR systems are AA copolymers neutralized with ammonia or dimethyl ethanol amine (DMEA). The OH content is from 2.0 to 4.8%, non-volatile content is from 40-45%; there is further 0 to 10% organic co-solvent as butyl glycol (BG), butyl diglycol (BDG) pure or admixed with naphtha (N). Viscosities are between 200 and 1,500 mPa.s.
There are several methods for the conversion of hydroxyl end groups into amine end groups, of which among the more commonly used is the reductive amination of secondary hydroxyl groups of polypropylene glycols. These specialized polyamines (amine-terminated polyethers) (Figure 1.34) are aliphatic in nature (making them suitable for both aliphatic and aromatic polyurea elastomers) and possess hindered amine groups. Given their flexible nature, polyetheramines include the soft-block segments of a polyurea chain. In addition to the polyetheramines, other low-molecular weight polyamines are employed as chain extenders. They react with the isocyanate component very fast, forming polyurea used in extremely fast processes such as RIM (reaction injection moulding) (Chapter 4) and elastomeric RIM-spray coatings (Chapter 7). The polyurea spray elastomer technology was introduced into the marketplace in the late 1980's, and is finding wide acceptance in a variety of commercial applications such as coating of concrete and secondary containment floorings. Moisture or low ambient temperature does not affect the very reactive and non-catalyzed polyurea systems. They have excellent adhesion and can be formulated into 100% solid systems so as to attend environmental regulations. These products exhibit excellent mechanical properties and durability even under adverse ambient conditions.
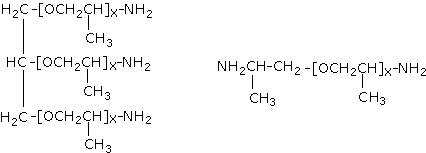
Figure 1.34 - Polyether amines
Chain extenders, curing agents, and crosslinkers are low molecular weight polyols or polyamines used to improve PUs properties. Normally, chain extenders and curing agents are used in flexible PUs as flexible foams (Chapter 3), microcellular elastomers (Chapter 4.8), casting elastomers (Chapter 6.2), polyureas (Chapter 4.10), adhesives (Chapter 7.1) and coatings (Chapter 7.3). Alcohols react with diisocyanate to form polyurethane hard segments and with amines to form polyurea hard segments. Those hard segments segregate, resulting in modulus increase and higher glass transition temperature (Tg). The higher density of hydrogen bonds of polyurea hard segments is responsible for the improved mechanical properties of polyurea and polyurethane/urea products.
1.5.1 - Hydroxylated chain extenders
Usually, chain extenders are difunctional compounds, such as glycols used in PUs, and diamines or hydroxylamines used in polyureas and polyurethane/ureas. Cure agents or curative are used in two-step processes for cast elastomers (prepolymer processes), and normally are chemically similar to chain extenders. Crosslinkers or crosslinking agents are tri- or polyfunctional compounds used to increase reticulation in rigid (Chapter 5) and semi-rigid (Chapter 4.5) foams. Diols are used as chain extenders in one-step and in two-step (prepolymers) processes. Table 1.11a shows hydroxylated compounds used as chain extenders or crosslinkers.
Table 1.11a - Hydroxylated chain extenders and crosslinkers|
Compound |
Structure |
Functionality |
MW |
|
HOCH2-CH2OH |
2 |
62 |
|
|
HOCH2-CH2-O-CH2CHOH |
2 |
106 |
|
|
|
2 |
76 |
|
|
|
2 |
134 |
|
|
HOCH2-CH2-CH2-CH2OH |
2 |
90 |
|
|
HOCH2-CH(CH3)-CH2OH |
2 |
90 |
|
|
water |
HOH |
2 |
16 |
|
|
2 |
221 |
|
|
|
2 |
198 |
|
|
HOCH2CH2NHCH2CH2OH |
3 |
105 |
|
|
N-(CH2CH2OH)3 |
3 |
149 |
|
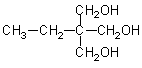 |
3
|
134
|
|
|
HOCH-CH2OH-CH2OH |
3 |
92 |
1.5.2 - Diamines used as chain extenders
Low molecular weight diamines (Table 1.11b) are used as chain extenders in polyurea and polyurethane/urea processes. They react much faster with isocyanates than with polyols (Table 1.3). Due to their longer pot life, the less reactive aromatic diamines are used in cast PU elastomers prepared in two-step processes (Chapter 6.2). Aliphatic and aromatic amines are used as chain extenders in polyurea RIM processes (Chapter 4.10) and spray coatings (Chapter 7.3.6), where their higher reactivity results in shorter demold times. The use of more reactive aliphatic, or less reactive secondary aromatic diamines makes possible to vary the profile of the system reactivity. Aliphatic diamines such as hydrazine or ethylene diamine are used as chain extenders in processes directed to preparing PU aqueous dispersions (Chapter 7.3.5). Cyclo-aliphatic diamines are applied with aliphatic isocyanates in spray coatings to prevent the yellowing occurring with aromatic PUs.
Table 1.11b - Diamines used as chain extenders|
Compound |
Structure |
MW |
|
H2N-NH2 |
32 |
|
|
Ethylene diamine |
H2N-CH2-CH2-NH2 |
60 |
|
1,4-ciclohexanediamine |
|
114 |
|
|
170 |
|
|
4,4’-bis-(secbutilamine) diciclohexylmethane (SBADCHM) |
|
322 |
|
4,4’-bis-(secbutilamine) diphenylmethane (SBADFM) |
|
310 |
|
diethyl-toluene diamine (DETDA) isomers 2,4 (80) e 2,6 (20) |
|
178 |
|
|
267 |
|
|
4-chloro-3,5-diamino-benzoic acid isobutylester (CDABE) |
|
242,5 |
|
3,5-dimethylthio-toluenediamine (DMTDA) - isomers 2,4 (80) e 2,6 (20) |
|
214 |
|
trimethyleneglycol-di-p-aminobenzoate (TMGDAB) |
|
314 |
|
4,4’-methylene-bis-(3-chloro-2,6-diethylaniline) (M-CDEA) |
|
365 |