Particulate and fibrous fillers (Table 2.9) may each be used in most kinds of PU. There are many reasons for adding fillers. Particulate fillers are used in flexible PU foams to increase the weight and resistance to compression and to reduce their flammability. PU foams, which contain fillers, represent a well-established foam technology. Fillers used in foam formulations can be inorganic in nature, but it is difficult to produce a stable suspension, and for this reason, organic fillers are usually preferred.
Flexible foams employing organic fillers like styrene-acrylonitrile copolymers or PHD polyols meet fire performance specifications for bedding and furniture applications and, with melamine as flame retardant material, also pass ignition source tests. For low-density foam, it is better to use melamine consisting of fine particles (10 mm). In this case, the flame retardant effect is enhanced by improved distribution of the melamine incorporated into the foam cell structure. In general, lower density foams require higher levels of melamine to pass the flame retardancy test. Inorganic solid materials used in filled foam formulations include: hydrated alumina, carbonates, silicates, silica, glass fibers, and barium sulfate.
Historically, inorganic fillers such as barium sulfate and calcium carbonate have been used in flexible slabstock foams to achieve increased density and/or load bearing, and to reduce cost. Normal used concentrations range from 20 to 150 parts per hundred parts polyols. The use of inorganic fillers has several disadvantages including: difficulty of preparing and maintaining the dispersion; problems with removal of entrained air; difficulty of mixing and pumping the filler/polyol slurry; loss of the foam physical properties; difficulty of processing on all types of foam machinery, and, due to their abrasive nature, increased wear on machinery components.
Table 2.9 - Fillers and their applications in PU|
Filler |
Typical applications |
|
Calcium carbonate (ground chalk, ground limestone, whiting) |
Flexible foams, semi rigid foams, binder compositions, rigid integral skin foams. |
|
Barium sulphate (barytes) |
Flexible foams, semi rigid foams, especially for sound absorption. |
|
Clays (china clay, kaolins, etc) |
Flexible systems. |
|
Expanded silicas, colloidal silicas |
Flexible foams, cast elastomers. |
|
Clay balls, vermiculite |
Rigid foams.. |
|
Glass micro spheres |
Flexible, microcellular foams, RIM. |
|
Glass flakes |
Elastomeric RIM. |
|
Silicates, cements |
Rigid foams, sealants, grouting. |
|
Short fibers, milled and chopped glass fiber |
Elastomeric RIM, rigid foams. |
| Glass cloths and scrims, wire mesh, organic fibers | Encapsulation in rigid foams, reinforcement of low-density flexible foam moldings. |
Mineral fillers are also used to reduce costs, and to increase the compressive strength of rigid foams used in composite building panels. Finely divided fillers with a particle size ranging from a few microns up to about 100 microns are usually added as dispersers in the polyol component of the PU systems. Some low cost mineral fillers such as china clay, kaolins, and other aluminium silicates, which contain both free and combined water, which would otherwise be satisfactory, may be difficult to dry reproducibly and economically. Care must be taken to dry the fillers or to known the precise water content available and factor that data into the foam calculations. Fibrous fillers are reinforcers: they give increased stiffness, and they increase the range of operating temperatures of rigid foams, integral foams and flexible RIM products. The degree of reinforcement obtainable depends on the strength of the fiber, the concentration, the modulus and extensibility of the polymer matrix, the interfacial adhesion and the shear strength at the fiber/polymer interface, and on the orientation of the fibers.
Like most polymeric materials the PU's are also susceptible to aging and the physical properties are normally negatively influenced. For example: PU's are subject to degradation by free radical pathways induced by exposure to heat or ultraviolet light; Polyester based PU's are more susceptible to hydrolysis (Chapter 1). However, the surface yellowing of PU based on aromatic isocyanates due to its exposure to light is merely an esthetic aging effect, which produces no loss of its mechanical properties. Light protection agents, such as hydroxybenzotriazoles, zinc dibutil thiocarbamate, 2,6-ditertiary butylcatechol, hydroybenzophenones, hindered amines and phosphites have been used to improve the light stability of PU's.
Degradation of both the polyol and urethane components will cause changes in the physical or mechanical properties of the PU's. Urethanes are susceptible to degradation by free radical pathways induced by exposure to heat or ultraviolet light. Autoxidation (Figure 2.16) may be initiated by heat, high-energy radiation UV (UV light), mechanical stress, catalyst residues, or through reaction with other impurities. Free radicals are generated (step 1) which react rapidly with oxygen to form peroxy radicals (step 2). These peroxy radicals may further react with the polymer chains leading to the formation of hydroperoxides (step 3). On exposure to heat or light, hydroperoxides decompose to yield more radicals that can reinitiate the degradation process (stage 4).
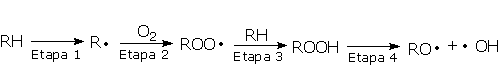
Antioxidants interrupt the degradation process in different ways according to their structure. Primary antioxidants, mainly acting in step 1 as chain breaking antioxidants, are sterically hindered phenols. Primary antioxidants react rapidly with peroxy radicals (ROO·) to break the cycle. Secondary arylamines, another type of primary antioxidant, are more reactive toward oxygen-centered radicals than are hindered phenols. Synergism between secondary arylamines and hindered phenols leads to regeneration of the amine from the reaction with the phenol (Figure 2.17).
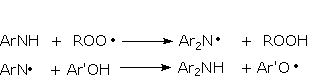
Phospite stabilizers are secondary antioxidants (hydroperoxide decomposers). Acting in step 4, they react with hydroperoxide (ROOH) to yield non-radical, non-reactive products. Secondary antioxidants are particularly effective in synergistic combination with primary antioxidants.
Hindered amine stabilizers (HAS) can in some cases, provide radical trapping effectiveness similar to hindered phenols. Traditionally used as light stabilizers, hindered amine stabilizers can also contribute to long-term thermal stability.
PU's are subject to degradation when exposed to natural and/or artificial UV lights. Degradation results in discoloration and/or loss of physical properties. In the photo-degradation mechanism, initially the polymer absorbs UV radiation, which excites the absorbing species, and raises them to a higher energy level (R to R*). If the molecule cannot be brought to its ground state, homolytic bond cleavage and the formation of free radicals will occur (R* to R·). The free radicals formed during photolysis readily react with oxygen to form peroxy radicals (ROO·), and the subsequent steps are similar to those in Figure 2.15. There are two classes of light stabilizers: 1) UV absorbers protect against photo-degradation by competing with the polymer for the absorption of ultraviolet light; 2) The mechanism of stabilization of hindered amine light stabilizers (HALS) involves efficient trapping of free radicals with subsequent regeneration of active stabilizer moieties.
Depending on the type of antioxidant used, the addition of antioxidants may also result in several undesirable side effects. Phenolic antioxidants (especially BHT) have long been known to migrate into fabric coverings, and, under certain conditions, to cause staining. The yellowing occurs when basic fabric finishes or basic cleaners are used. Acid finishes and rinses together with sunlight can reduce or eliminate the color. Secondary amine antioxidants are thought to be significant contributors to discoloration. Thioesters can react with oxides of nitrogen (exhaust fumes) to form orange to red color bodies. Finally, certain phospite antioxidants suffer from hydrolytic instability and can decompose to deleterious phosphoric acid during extended storage in polyol solutions.
Most low-density flexible foams are color-coded during manufacture to identify the grade and the density of the foam. Specialized products, such as foams for textile laminating and for packaging, may be highly colored to meet the requirements of the application. On the other hand, rigid foams, being mostly made from brown-colored polymeric MDI and sold enclosed within opaque covering materials, are often made without added colorants. The usual method of coloring adds pastes, made with polyols and inorganic or organic pigments, to the foam reaction mixture. Typical inorganic coloring agents included titanium dioxide, iron oxides and chromium oxide, organic pigments originated from the azo/diazo dyes, phthalocyanines and dioxazines, as well carbon black. Typical problems encountered with these colorants include high viscosity, abrasive tendencies, foam instability, foam scorching, migrating color and a limited range of available colors. The most popular coloring material is carbon black, which at levels above 0.1 parts per 100 parts of the polyol used gives some protection against the surface discoloration of the foam caused by UV light.
Polyurethanes based on aromatic isocyanates tend to yellow on exposure to daylight. Thus, to screen uncovered items such as self-skinning foams, microcellular molding and RIM products from UV light, carbon black pigmentation, or UV light absorbing additives must be used as protectors (Chapter 2.6). Another method of coloring is the surface coating of the finished PU. This is performed by subsequent painting of PU parts with light stable lacquers, preferably one-component PU lacquer systems based on blocked isocyanates, or two-component systems (Chapter 7). Two approaches are commonly used: first, in order to reduce the number of operations and to simplify any difficulties associated with the demolding of the product and with paint adhesion, in-mould coating is widely used. According to this procedure the coating is sprayed onto the mold surface before the PU reaction is carried out; then, flexible or rigid topcoats of PU are applied after demolding, to provide a high degree of abrasion resistance.
Given the application of sufficient heat in the presence of oxygen, PU's, as all organic materials will burn. The physical state of the polymer is also extremely important. Low-density, open-celled flexible PU foams have a large surface area and high permeability to air, and, thus, will burn most easily. Flame retardants are often added to reduce this flammability, at least, when they are to be measured by various specific, often small-scale tests, conduced under controlled laboratory conditions. The choice of flame retardants for any specific PU foam often depends upon the intended service application of that foam, and the attendant flammability-testing scenario governing that application.
Aspects of flammability that may be influenced by additives include the initial ignitability, burning rate and smoke evolution. On flame retardants, manifold requirements are placed: They should be compatible with the mixture of raw materials and additives and should not be able to migrate out of the finished products. In addition, the mechanical properties of the finished products should be affected as little as possible and, in case of burning, they should form little smoke and no toxic fumes.
In PU flexible foams, the most widely used flame retardants are the chlorinated phosphate esters. Chlorinated paraffin and melamine powders have also been used (Table 2.10). However, the incorporation of flame retardants in foams can present problems such as: to increase the possibility of burns in certain formulations; to increase the amount of smoke or burns; and to cause processing problems, especially with the reactive flame retardants.
According to theory, halogen compounds work in the gas phase, interrupting the free radical nature of the combustion process. They work either as molecules or interfere as halogen atoms, formed through their burning, in chain reactions. The phosphorous compounds effect a catalytic splitting of the PU and lead through dehydrogenation and dehydration reactions to a carbonized, protective surface. Technical important synergistic working combinations are halogen compounds / antimony trioxide, phosphorous compounds / halogen compounds and phosphorous compounds / nitrogen compounds.
Table 2.10 - Some flame retardants for PU|
Flame retardants |
Main application |
|
Non-reactive liquids |
|
|
Tris(1,3-dichloroisopropyl) phosphate (TCPP) |
Flexible foams |
|
Tris(2-chloroisopropyl) phosphate |
All PU foams |
|
Tris(2-chloroethyl) phosphate (TCEP) |
Flexible foams and coatings |
|
Pentabromodiphenyl oxide |
Flexible foams |
|
Tetrakischloroethyl-2,2-bis(chloromethyl)propylene-diphosphate |
Flexible, rigid and molded foams |
|
Tris(2,3-dichloropropyl) phosphate (TDCP) |
Strip or block flexible foams |
|
Reactive flame retardants |
|
|
Dibromopropanol |
|
|
Diester/ether diol of tetrabromophthalic anhydride |
Rigid foam, elastomers and coatings |
|
Tetrabromophthalate diol |
Rigid foams, RIM, elastomers, adhesives, coatings and fibers |
|
Tetrabromophthalic anhydride |
Rigid foams |
|
Solid flame retardants |
|
|
Antimony Trioxide |
Synergistic effect |
|
Ammonium salts, sulphate, polyphosphate, etc |
Together with halogenated additives in rigid foams |
|
Aluminum hydroxide |
All polyurethanes |
|
Melamine |
Flexible foams |
|
Calcium carbonate |
Heat absorbing filler |
|
Flaked or powdered PVC |
|
Solid flame retardants
Liquid flame retardants have always been preferred because other components for PU foam are liquid. However, solids, which are especially cost effective, have been in continuous use. Solids have the disadvantage of needing to be slurred with another component. Pumping and metering equipment life is shortened due to the abrasive nature of solids.
Flaked or powdered PVC is a soft and inexpensive flame retardant when comparatively mild flammability tests are required. There has been concern about the evolution of HCl when foam containing PVC is flame laminated. Ammonium polyphosphate is used with liquid halogenated phosphate esters in ester polyol based foams.
A combination of liquid and solid flame retardants often has a less deleterious effect upon physical properties than the same quantity of liquid or solid additive alone. In combustion modified high resilience, foams (CMHR) are often incorporated to the solids antimony trioxide, alumina trihydrate, and decabromodipheyl oxide as well as to a liquid halogenated phosphate ester. The presence of both chlorine and phosphorous is necessary for the optimum effect upon flammability.
The addition of aluminium trihydrate gives further reduction in flammability, and minimizes the increase in smoke formation on burning, resulting from the addition of halogenated organic phosphates. Aluminum hydroxide, between 180 and 200oC, splits off water and changes to aluminum oxide. Its effectiveness depends on the fact that endothermic splitting of water withdraws heat from the system, the formed water vapor dilutes the gas formed by polymer cracking, and the aluminum oxide forms an insulating protective layer. Melamine is lower in cost, less dense, and effective when used together with phosphate flame retardants, such as ammonium polyphosphate, in flexible foams for furniture cushions.
Melamine melts away from the flame and forms both a nonflammable gaseous environment and a molten barrier that helps isolate the combustible PU foam from the flame.
Liquid flame retardants
Due to their efficiency, compounds containing aliphatically bound halogen or combinations of aliphatically bound halogen and phosphorous were among the first flame retardant compounds used. Many ignitability standards for furniture and vehicle seating can be met by the incorporation of 5% to 10% chlorinated phosphate esters. Many of the most efficient flame retardants (Table 2.11) are no longer in use commercially due to toxicity concerns or to their effect upon foam properties.
Table 2.11- Flame retardants efficiency|
Flame retardant |
Level required (php) |
|
Tribromoethanol |
5 |
|
Dibromopropanol |
5 |
|
Tris(dibromopropyl)phosphate |
6.5 |
|
Bischloroethylethylphosphonate |
6.5 |
|
Tetrakischloroethyl-2-2-bis(chloromethyl)ethylene-diphosphate |
7.5 |
|
Hexabromocyclododecane |
7.5 |
|
Tetrakischloroethyl-2,2—bis(chloromethyl)propylene-diphosphate |
10 |
|
Tris(dichloropropyl)phosphate |
10 |
|
Tris(chloroethyl)phosphate |
10 |
The most used flame retardants in both flexible and rigid foam systems are chlorinated phosphate esters. These have a significant effect upon the ignitability of foams by a small heat source, and they may show marked reductions in the rate of burning in small-scale tests without adverse effects upon the processability of the foam system and the properties of the product.
Liquid flame retardants represent the largest volume of flame retardants used in flexible PU foam, but despite their ease of handling and their compatibility with other liquids, they have inherent problems. One of these is volatility, Internal temperatures in large burns can reach 160oC or higher during cure. Volatile, low molecular weight liquids are capable of migrating during foam cure, affecting the flammability of the burn center. The introduction of aging criteria to many flammability test standards has prevented this problem.
An even greater problem related to the exothermic of curing PU foam is the discoloration caused by inadequate thermal stability of liquid flame retardants. This discoloration or scorch is most prevalent when aliphatic halogen containing flame retardants are used. It has been suggested that acidity due to dehydrohalogenation catalyses the oxidation of the polyol.
The role of aromatically bound bromine is scorch reduction, and aromatic bromine containing flame retardants have been used in PU flexible foams to prevent scorch. The hydrohalogen acids formed reduce IFD (indentation force deflection) spread in HR foams. IFD spread is observed when dibutyltin dilaurate (DBTL) is used as a catalyst. It is believed that the acid formed inactivates DBTL, which can promotes depolymerization of the PU foam.
Hydrolytic decomposition of flame retardants may also be a problem, particularly in polyester PU foam subjected to humid aging at elevated temperature. Another property affected by flame retardant hydrolytic stability is the final cure rate. Acidic species generated neutralize the tin and amine catalysts, slowing cure.
The outstanding adhesion of PU to other material has led to a broad application in the adhesive sector (Chapter 7). This property is detrimental in foam molding. A release agent is necessary in order to easily and quickly remove the foam from the mold. The effectiveness of the release agent depends less on the amount than on the uniformity of the coating. The force per area necessary to open the mold can be used as scale for the release effectiveness. In order to choose the best release agent, a basic knowledge of the PU system and of the kind of mold material, surface quality and form geometry are decisive. The adhesion to the mold surface decreases with increasing reactivity and increasing density of the reaction mixture, due to the shorter period of wetting by the isocyanate component.
The release agent is best applied by spray coating the open mold. Sufficient ventilation must be provided. In general, the removed parts must be subjected to an after treatment to remove adhering release agent residues. This measure is indispensable if the finished part is to be painted afterwards. Removal of the release agent residues from the mold must be done after every one or two production shifts. This is done with solvents, such N-methylpyrolidone (wiping with soaked rags), or with cleaners offered by the release agent manufacturers (in the form of sprays, liquids or pastes).
There is no universally applicable alternative, and the best release agent can only be found by testing under production conditions. Several techniques are available to avoid the application or removal of release agents in particular areas:
Internal release agents - From the point of view of the processor, the ideal process only uses internal release agents, which are used rather successfully in RIM formulation, added to polyol component. The use of external release agents, however, remains a necessity. Normally, it is possible to achieve 20-100 demoldings until the external mould release agent has to be applied again. Metallic soaps such zinc stearate, ester based oils, waxes, and siloxanes are examples of products in use. These substances are insoluble in the resins and, therefore, they have to be distributed homogeneously. During the PU formation, these emulsions or dispersions beak respectively, the active ingredients migrate to the surface and, there, they form a very fine thin film. This film acts like a barrier and prevents the formation of physical and chemical interactions between the foam and the mould, finally providing a releasing layer for demolding the part. Siloxanes, in use as internal mould release agents, are normally potent denucleating additives or even defoamers, as well. They reduce the nucleation considerably so that the possible air-load comes down do only 20%. Internal mould release agents based on pure organic substances do not have this drawback. On the other hand, they are less effective. Disadvantages may occur during possible after treatment of the finished part because the release agent may still migrate to the surface even after painting, and lead to flaking of the paint.
Mould coating - The use of permanent mould coatings, such as PTFE coatings, increase substantially the productivity by reduction of the release agent application time, but it has some disadvantage. This is due to the limited life and difficult renewal. A further disadvantage is the smaller possibility of controlling the surface gloss of the finished part. Semi-permanent release agents are also used in many sectors. Polysiloxanes may polymerize on the mould surface, and, thus, produce a release reserve for several removals from the mould. The disadvantage may lie in the different surface of the finished part. The first molding, removed immediately after application of the release agent, often has different characteristics from the last molding, which can be removed only with difficulty. Semi-permanent release agents often act successfully in conjunction with internal release agents. Quickly reacting RIM systems frequently operate as so-called easy release systems, i.e., with and internal release agent in conjunction with an external semi-permanent application. This may be a hard wax, which is polished on the mould surface. The technical standard in most cases is the use o a hard soap, which does not have as long a life as a polished wax, but which can be applied far more quickly with a spray gun.
External mould release agents - External release agents, which are applied on the moulds using different techniques have to be very incompatible with the processed materials. Furthermore, they should have a low surface tension, to allow the formation of closed, homogeneous and very thin films on the surfaces with low energies. These films should not have any reactive groups; they should be chemically inert. However, a few polar groups are necessary to achieve a sufficient substantivity of the release substances on the moulds. Siloxanes, as external release agents, are preferred for microcellular systems, particularly for shoe soles. They meet the aforementioned conditions in an ideal way. The portion of polar groups necessary for the appropriate substantivity can be achieved by modifying the siloxanes with organic side groups. Besides good release, silicone based release agent provide good compatibility with coloring materials used for shoe soles. As the films are liquid, the formation of a hard buildup in the molds is not possible. The viscosity of the siloxanes may be reduced to such an extent that they become volatile. The result is that the surface of the mold is nearly free of any release agent. This technique is especially successful for polyesterpolyurethanes.
The external mould release agents for processing RIM systems are based either on waxes or metallic soaps. Aqueous solutions of metallic soaps are preferred whenever metallic soaps are applied as internal release agents. It is recommended that wax based external release agents be used when the internal release agent is a siloxane type one. Combinations of waxes with siloxanes are useful for integral skin foams, to provide surfaces with a silky finish. The use of solvents, in particular the ozone depleting halogenated hydrocarbons and CFC's, has become severely restated by several clean air acts.
Water based release agents - In global terms, they are the most common as they comply with the environmental norms. They have as disadvantage higher cost, higher water boiling point, and their reactivity with isocyanates, that can cause skin formation. In the case of molded flexible foams, the skin formation (closed cells) increases the hardness. The use of water based release agents has been the state of the art for several decades in the field of hot foamed material production, thanks to the high mould temperatures and the resulting-free evaporation of the release agent. The disadvantageous physico-chemical properties of water compared to other carriers such aliphatic hydrocarbons, chlorinated hydrocarbons, or CFC's were an important obstacle to the use of these systems in other PU fields.
Solvent based release agents - They constitute one of oldest technologies in use, and they are still the most commonly used in cold molded flexible foams. In them, a small amount of wax is suspended in a low boiling point solvent, as petroleum spirit, methylene chloride, etc. When the release agent is sprayed into the hot mold, the low boiling point solvent evaporates, resulting in a film formation in the mold surface that prevents the adherence of PU. Chlorine containing products such as methylene chloride are rejected for workplace and environmental protection reasons, although methylene chloride, unlike 1,1,1-trichloroethane and CFC 11, has not been banned so far. Only the pure hydrocarbons in their fractions then remain as suitable carriers for release agents. The reason lies in the favorable prices compared to chlorinated solvents. Important disadvantages such as flammability and the slower evaporation rates have been tolerated. A dearomatised white spirit with a flash point of about 25oC has established itself as carrier material. The low-solids solvent-based release agents possess 97% of solvent, which is liberated in the atmosphere during the application, and have also high VOC (value of oxygen consumption).
High-solid release agents - The solvent-base high-solid release agents have smaller VOC than low-solid solvent-based release agents, due to the reduction of the solvent level to 85-92%. If we consider the application of an equal amount of release agent, we would have a theoretical reduction of 70-80% in VOC. Though, in practice, it is very difficult to control the application level and the excess used results in increase of VOC. In the case of the water-based high solids release agents, a part of the hydrocarbon-based carrier was replaced by water, but by maintaining the properties of normal high-solids. However, this is only recommendable if the solvent emissions have to be reduced once again, when compared to normal high-solids.
Pastes - The history of release agents in PU industry began with pastes, such as floor waxes, which are still widely used today. This applies in particular to the application of prime coatings to the moulds. However, pastes with the most diverse compositions and hardnesses are also used in many applications in which the evaporation time is not a problem. The organic solvents have been replaced by water in many sectors. Many water-based release agents are used only in conjunction with water-based pastes as mould primers. Solvent-based mould primers often cause defects on the finished part.
Besides the previously described auxiliary agents are also added special addictives to promote properties in PU's. For example, coatings, adhesives, sealants and the variety of topcoats, which have to be glued, require a large number of these special addictives.
Organo silanes are useful as crosslinkers for PU sealants and adhesives (Chapter 7). They react with the NCO terminated PU prepolymer to form an endcapped silane prepolymer, call silylated PU (SPUR). The SPUR's based sealants and adhesives react fast with moisture at room temperature, have good durability, excellent adhesion performance and they are free from any unreacted isocyanate residual monomer. They can be formulated with a wide range of addictives, as fillers, plasticizers, adhesion promoters, moisture scavengers, etc.
Adhesion promoters are used to provide a reaction in the surface of the substrate and by this way to promote the adhesion, providing a bond between the PU's and the substrate. They can be applied in the surface of other materials, such as metallic parts. Adhesives and sealants of PU frequently can be combined with organo silanes (aminosilanes, mercaptosilanes, epoxysilanes, etc) that form a chemical bond between the adhesive or sealant resin and the substrate. This chemical bond is resistant to moisture, chemical products, and heat.
Silanes chemically bond organic polymers to inorganic material such glass fibers, glass spheres, silica, titanium dioxide, clays, metal and metallic oxides. Beyond providing a means of bonding inorganic filler to a PU resin, improving filler and pigment dispersion.
Water bonding agents are added for increasing the storage stability of one and two PU component systems. In PU solid systems, besides the careful drying of all components the addition of a drying agent (2 to 4%), like zeolite or molecular sieve, to the polyol component is recommended to bond possible residual moisture. Zeolite pastes ensure a quick and excellent dispersion in the PU and are, in addition, less sensitive ho humidity than zeolite powder. Silanes, especially vinyl silanes, have found wide use as moisture scavengers. The electro-withdrawing nature of the vinyl group, furthers enhances the water reactivity of the silicon-methoxy bond. As moisture scavengers it reacts with moisture faster than other alkoxy silanes, enabling it to function as a moisture scavenger in the presence of the other silanes incorporated as adhesion promoters, crosslinkers or coupling agent. A nominal amount (3% pbw) of vinyl silane is required to enhance the shelf-stability of a moisture sensitive product.
Rheology modifiers are used for control of the rheology by the reduction of the fluidity, avoiding the flowing of adhesives, selantes, paints, etc, and they include: pyrogenic silica, betonites and carbon black. The addition finely divided silica can prevent the no wanted penetration of adhesives in porous materials as leather, textile and concrete. The silicas (silicon dioxide) of extremely small particle size are used to modify the rheology of liquid systems. It is know as highly disperses, colloidal, or still fumed silica due to it production process. They find application mainly, in the modification of the rheology of PU sealants, adhesives, paints, coatings, etc.
Non-reactive liquids have been used to soften a PU or to reduce viscosity for improved processing. Plasticizers as the phthalates, benzoates and chlorinated paraffins are used for reduction the viscosity and cost, however they reduce the PU mechanical properties like tensile strength and hardness, and also decrease the PU glass transition temperature (Tg). Using a polyol of lower equivalent weight can compensate the softening effect so that a higher cross-linked polymer structure is obtained.
The PU's are used in some packing and clothing applications as safety shoes, packing electronic goods, etc, that's requires a strong decrease of electrical resistance. The reduction of electrostatic charge build-up is attained through antistatic agents, for example tetra alkyl-ammoniumalkylsulfates. They are mixed with the polyol component or added to the isocyanate reactant and diminish the surface resistance by approx. 108 Ohm. Some flexible PU foams are also used in packing, clothing and other applications where it is desired to minimize the electrical resistance of the PU foam so that buildup of static electrical charges is minimized. This has traditionally been accomplished through the addition of ionizable metal salts, carboxylic acid salts, phosphate esters and mixtures thereof. These agents function either by being inherently conductive or by absorbing moisture from the air. The desired net result in orders of magnitude reduction in foam surface resistivity.
In some PU foams it is necessary to add cell openers to obtain foam that does not shrink upon cooling. Known additives for inducing cell opening include silicone based antifoamers, waxes, finely divided solids, liquid perfluocarbons, paraffin oils, long chain fatty acids and certain polyether polyols made using high concentration of ethylene oxide.
Waxes, soaps and other products are added in small amounts as lubricants and oiling agents. They usually improve the flow characteristics of fluid reaction mixtures by lowering the viscosity and facilitating the extraction of PU molded part. Lubricants act as processing aids for injection molding and extrusion of TPU's (Chapter 6.3).
2.10.10 - Hydrolysis stabilizers
Polyester based PU's are subject to aging. The ester bonds can be hydrolyzed under the influence of humidity and higher temperatures. Polyester based PU's are stabilized against hydrolytic degradation by adding 1-4% by weight of sterically hindered aromatic carbodiimides. The carbodiimide group reacts with acid residues, generated by the hydrolysis of ester groups, which otherwise catalyze further hydrolysis.
Under certain conditions of warmth and high humidity, PU foams are susceptible to attack by microorganisms. When that is a concern, additives against bacteria, yeast or fungi are added to the foam during manufacture.
3 - Flexible Foams